Part:BBa_K4150004
His-OVA
ThisThe chicken ovalbumin sequence obtained from KEGG database (Gallus gallus) was codon optimzed for expression in E. coli and synthesized by Integrated DNA Technologies, Inc. with a connection of a His tag and a GS linker at N-terminus. The ovalbumin (OVA) is a glycoprotein, which is a major component of chicken egg whites, and harbors immunogenic properties in vaccination experiments[1]. The recombinant OVA protein is readily purified in E. coli BL21 system driven by T7 promoter and triggered by IPTG induction.
Characterization
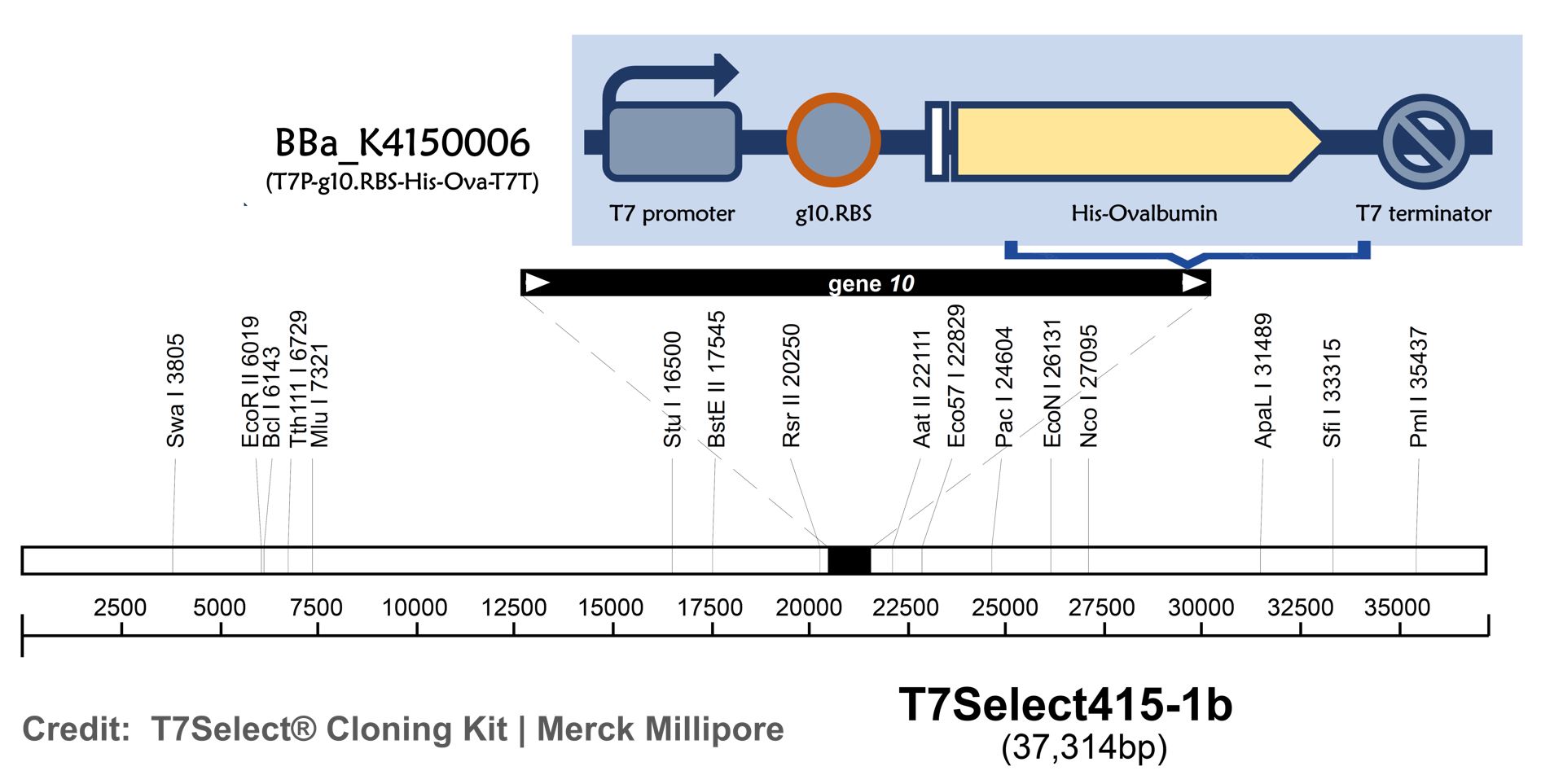
ThisT7 bacteriophage genome was edited with the BioBrick of T7P-g10.RBS-His-Ova-T7T (Part:BBa K4150006)in the end of T7 gene 10 using the T7Select® 415-1 Cloning Kit (Merck Millipore) according to the manufacturer’s instruction.
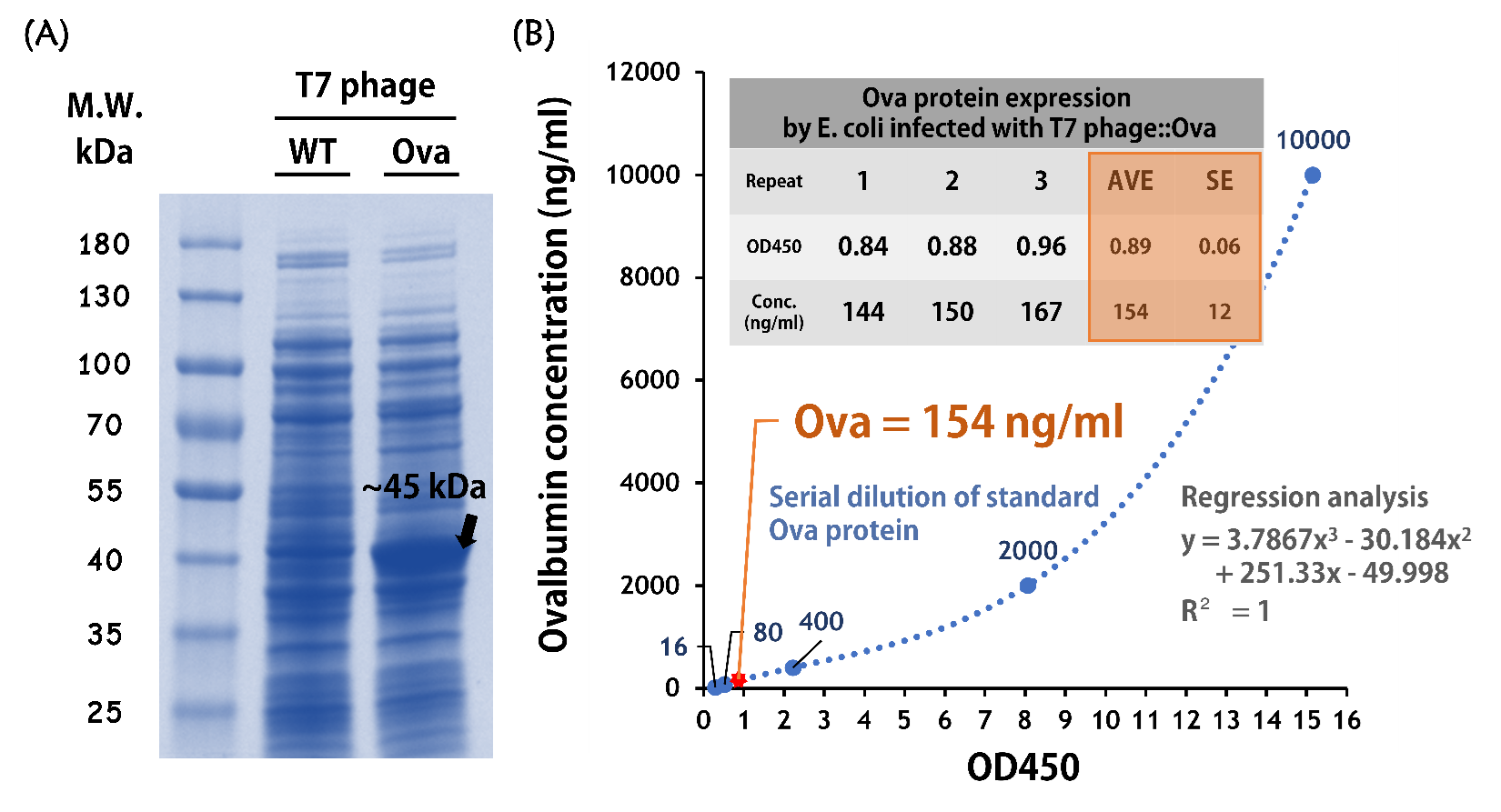
ThisTo test the Ova protein expression by the engineered phage, the isolated BALB/c mouse intestinal commensal E. coli were grown to an OD of 0.6 and infected with T7 phage::T7P-g10.RBS-His-Ova-T7T at a MOI of 5 for 2 hr at 37°C. The bacterial lysates were subjected to SDS-PAGE and Coomassie blue staining. The gel clearly showed a sharp band at the predicted size of a purified chicken ovalbumin protein (~45 kDa) compared to the lysates of E. coli infected with wild-type T7 phages (Fig. 9A).
ThisTo quantify the protein concentration produced by the engineered phage carrying Ova gene, 5 x 108 cells of the isolated E. coli (OD600 = ~0.6) were infected with 2.5 x 109 phages (MOI = 5) for 2 hr at 37°C. The 50 μl of lysates along with a serial dilution of standard of purified ovalbumin proteins were subjected to an ELISA Kit for Ovalbumin (CLOUD-CLONE CORP., Product No. CEB459Ge) and performed according to the manufacturer’s instruction. The data were measured at OD450 nm and determined by regression analysis due to the assay is based on the competitive inhibition enzyme immunoassay technique. As shown in the Fig. 9B, the concentration of Ova proteins produced in the E. coli infected with T7 phage::Ova were ~154 ng/ml (OD450 = ~0.89).
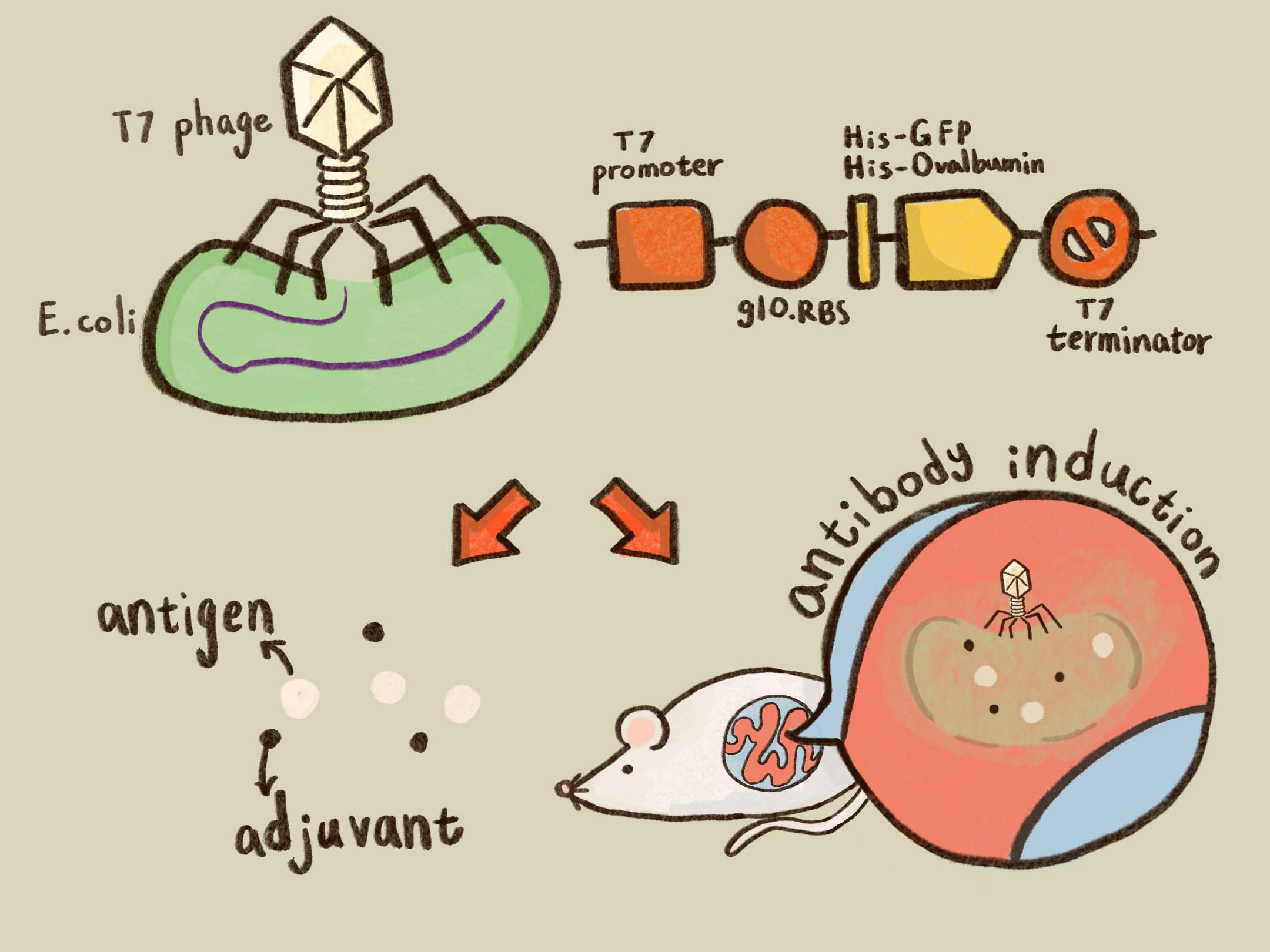
ThisTaken together, we successfully created and improved BioBrick parts, as well as engineered T7 phages, which are able to transfer genes (i.e., GFP and Ovalbumin) into mouse intestinal E. coli and produce proteins of interest by the infected E. coli.
Figure 9 | Ovalbumin protein production by the mouse intestinal E. coli infected with T7 phages::T7-g10.RBS-His-Ova-T7T. (A) 30 ng of total phage-infected bacterial lysates were run on SDS-PAGE with a 4–15% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad Laboratories, Inc.) and stained with Coomassie Brilliant Blue G-250. The PageRuler™ Prestained Protein Ladder, 10 to 180 kDa (Thermo Fisher Scientific Inc) was used as a marker. (B) 50 μl of the same lysates were also conducted in an ELISA Kit for Ovalbumin (OVA) (Cloud-Clone Corp.) along with a serial dilution of standard Ova protein provided in the kit. The values were read at OD450 and calculated by a formula made by a regression analysis.
Purification
ThisTo achieve our goal of building up the model antigen of phage ovalbumin induction system (i.e., phage – E. coli – protein production) for the application of vaccine testing, a His tag was fused at the N terminus of ovalbumin. We’d like to know whether the His-ovalbumin can be purified for future application.
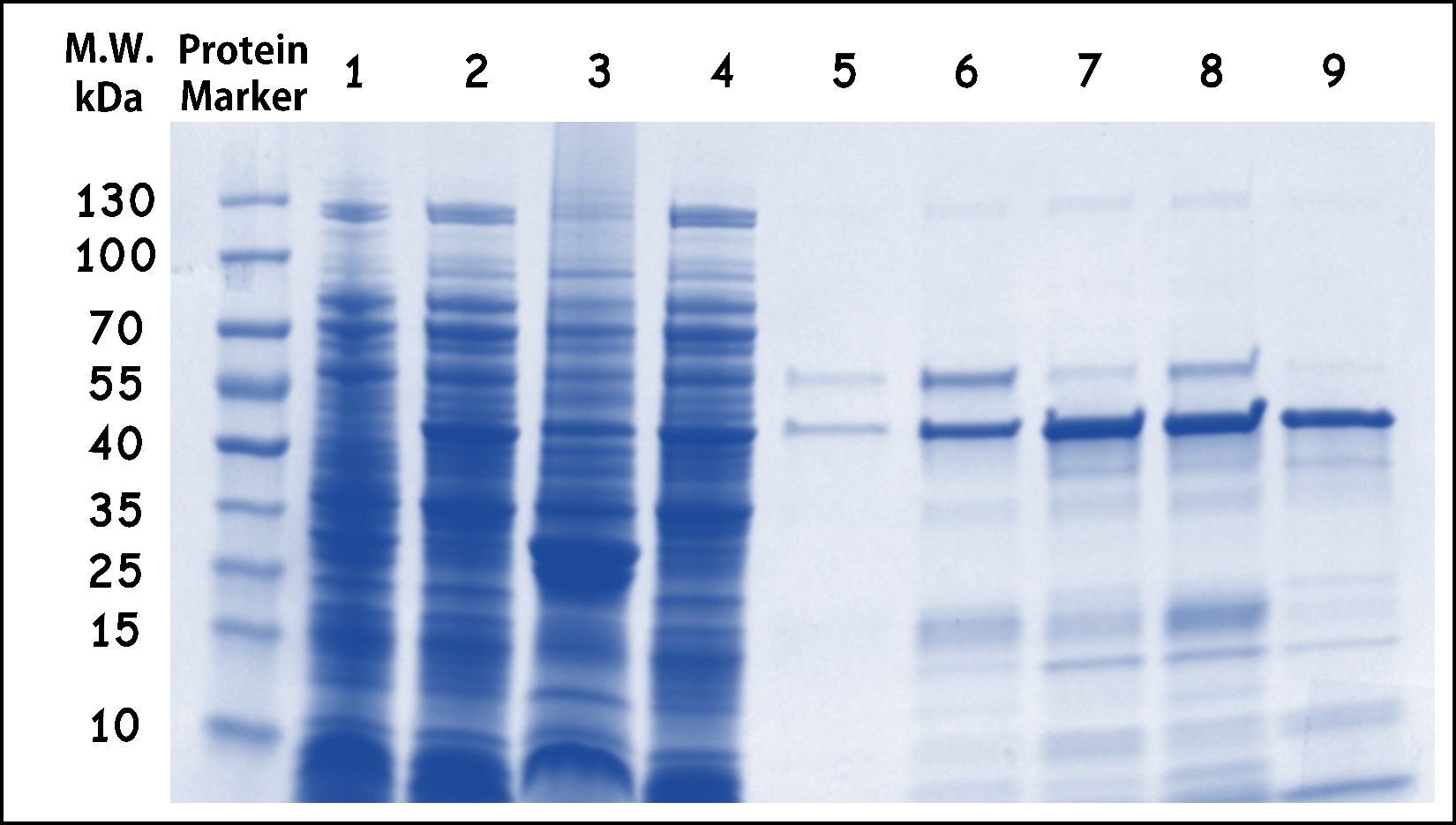
ThisThe His-ovalbumin protein from the lysates of E. coli DH5alpha infected with T7 phage::OVA was purified by Nickel column through the ÄKTA start protein purification system (Cytiva). The Fig. 10 showed the elutions contain predominantly purified His-ovalbumin proteins with a predicted molecular weight of 45kDa, although some degradation forms or impurities may leave in the elutions. Taken together, the ovalbumin protein in the E. coli infected by T7 phage:OVA can be produced and purified in our system. Therefore, the ovalbumin as a model antigen can be applied in the purified form or as phage-infected bacterial lysates.
Figure 10 | His-Ovalbumin purification from the phage-infected bacterial lysates. The lysates of E. coli DH5alpha infected with T7 phage::T7-g10.RBS-His-Ova-T7T were purified by Nickel column through the ÄKTA start protein purification system (Cytiva). The protein harvested were analyzed by SDS-PAGE and Coomassie Blue Staining using 4–15% Mini-PROTEAN® TGX™ Precast Protein Gels (Bio-Rad Laboratories, Inc.) PageRuler™ Prestained Protein Ladder was used as a marker. Lane: (1) E. coli control lysates without phage infection, (2) E. coli raw lysates with phage infection, (3) flow-through, (4) total lysates before purification, (5) wash-through, (6) Elution #8, (7) Elution #9, (8) Elution #10, (9) Elution #11.
Schematic Diagram for Application
Reference
- ↑ Geary TW, Reeves JJ. Production of a genetically engineered inhibin vaccine. Vaccine. 1996 Sep;14(13):1273-9. doi: 10.1016/s0264-410x(96)00014-x. PMID: 8961517.
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25COMPATIBLE WITH RFC[25]
- 1000INCOMPATIBLE WITH RFC[1000]Illegal SapI.rc site found at 373
| None |